Abstract
Zinc-ion hybrid supercapacitors (ZHSCs) are attracting significant attention due to their high energies/power densities, safety, and low cost. In this review, recent advances in the development of ZHSCs are summarized. Particular emphasis is placed on state-of-the-art cathodes (including carbon, metal oxides, MXenes, and redox-active polymers), anodes (including Zn-based composites and Zn-free materials) and electrolytes for ZHSCs. Furthermore, the latest research on functional ZHSC devices with miniaturized ZHSCs, fiber-shaped ZHSCs, self-chargeable ZHSCs and self-healing devices is reported. Finally, further developments with ZHSCs are envisaged for future research in this thriving field.
Introduction
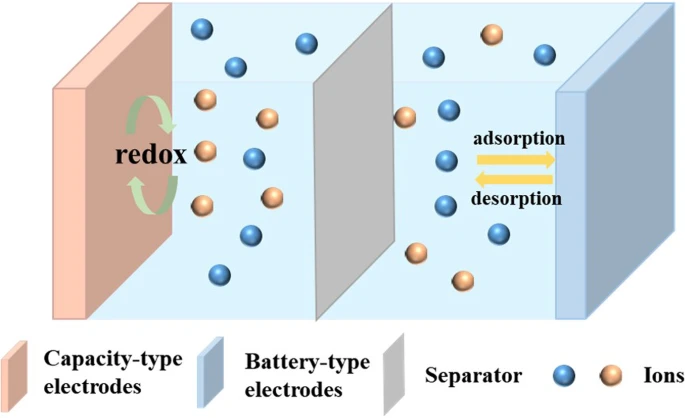
With the popularity and use of smart grids and electric vehicles, the demand for energy storage devices with high energy and power densities, excellent safety, and long cycling lives is increasing1,2. Rechargeable batteries and supercapacitors are widely researched and have been used for commercial applications3,4,5. Rechargeable batteries consist of two battery-type electrodes that store energy through redox reactions, such as ion intercalation/deintercalation, phase transformations, or stripping/plating at the anode and cathode; these batteries typically have very high energy densities and are mainly used in portable electronics and electric vehicles. However, the redox reactions in rechargeable batteries usually lead to short cycling lives and low power densities owing to volume variations and sluggish kinetics. Supercapacitors consist of two capacitive electrodes that store and convert energy through electrochemical double-layer capacitive or pseudocapacitive behavior; therefore, supercapacitors have high power densities and are used mainly in consumer electronics, backup power sources, renewable energy generation systems, rail transportation, military equipment, aerospace, and other fields, etc. However, the energy densities of supercapacitors are insufficient and need to be increased. For this reason, the concept of hybrid supercapacitors was developed. Hybrid supercapacitors combine the advantages of both batteries and supercapacitors by using capacitive and battery-type materials as electrodes. During charging and discharging, the capacitive electrodes serve as electrochemical double-layer capacitors or pseudocapacitors, and the battery-type electrodes carry out redox reactions (Fig. 1)5,6. When constructing hybrid supercapacitors, it is necessary to ensure that the supercapacitors have capacitive electrodes and battery-type electrodes. That is, hybrid supercapacitors can be classified into two types: one has a capacitor-type cathode and a battery-type anode; the other, on the contrary, has a battery-type cathode and a capacitor-type anode.
Among the various hybrid supercapacitors, nonaqueous lithium-ion hybrid capacitors were demonstrated early. However, considering the scarcity and safety issues associated with lithium resources and organic electrolytes, researchers have started to explore the prospects of other ion hybrid capacitors because of their lower costs and safety. Zinc (Zn) is very promising due to its high theoretical capacity (820 mAh g−1), low redox potential (−0.76 V vs. the standard hydrogen electrode (SHE)) and high reliability. Therefore, zinc-ion hybrid capacitors (ZHSCs), which combine the advantages of Zn-ion batteries, such as low cost, environmental friendliness, and low redox potentials of the Zn anodes, and the advantages of supercapacitors, including fast charge‒discharge rates, high power densities and long cycling lives, show attractive application prospects. Conventional ZHSCs use conventional carbon materials as the cathodes, commercial Zn foils as the anodes, and aqueous Zn salts as the electrolytes. Conventional carbon materials, such as activated carbon (AC), exhibit capacitive properties, but their limited specific capacitance cannot be matched with those of Zn anodes with high capacities, resulting in a waste of resources7,8. On the anode side, the need to polish the surface oxide layer of commercial Zn foil may cause fine traces to form on the surface of the Zn foil, leading to the formation of dendrites during cycling, which can affect the cycling life9,10,11. In addition, the interactions between water molecules and the anions in water-soluble Zn salts (ZnCl2, ZnSO4, Zn(CF3SO3)2, and Zn(CH3COO)2) can affect ion adsorption/desorption or deposition/exfoliation, which may cause a range of issues, such as byproduct formation and the hydrogen evolution reaction12,13. Here, we present the latest cathode and anode materials, compare the effects of advanced electrolytes on the performance of ZHSCs, and summarize the prospects for application of new ZHSCs. Moreover, novel design strategies for functional ZHSC devices are highlighted. In addition, we provide our perspectives on the remaining challenges and future research opportunities for the development of electrode materials, electrolytes, and devices for ZHSCs.
For more information please follow the link: https://www.nature.com/articles/s41427-024-00537-9



