Abstract
Ammonium ion batteries are promising for energy storage with the merits of low cost, inherent security, environmental friendliness, and excellent electrochemical properties. Unfortunately, the lack of anode materials restricts their development. Herein, we utilized density functional theory calculations to explore the V2CTx MXene as a promising anode with a low working potential. V2CTx MXene demonstrates pseudocapacitive behavior for ammonium ion storage, delivering a high specific capacity of 115.9 mAh g−1 at 1 A g−1 and excellent capacity retention of 100% after 5000 cycles at 5 A g−1. In-situ electrochemical quartz crystal microbalance measurement verifies a two-step electrochemical process of this unique pseudocapacitive storage behavior in the ammonium acetate electrolyte. Theoretical simulation reveals reversible electron transfer reactions with [NH4+(HAc)3]···O coordination bonds, resulting in a superior ammonium ion storage capacity. The generality of this acetate ion enhancement effect is also confirmed in the MoS2-based ammonium-ion battery system. These findings open a new door to realizing high capacity on ammonium ion storage through acetate ion enhancement, breaking the capacity limitations of both Faradaic and non-Faradaic energy storage.
Introduction
The aqueous secondary batteries taking ammonium ions (NH4+) as charge carriers have captured tremendous attention in the sustainable energy storage research in recent years1,2, which offer several notable advantages over traditional metallic carriers like Li+, Na+, K+, Zn2+, and Mg2+: firstly, NH4+ exhibits fast diffusion ability in aqueous electrolytes owing to its small ionic size and light molar mass3; secondly, the utilization of NH4+ carriers ultimately eliminate the problems of dendritic growth which induces safety concerns in metal-ion batteries4; thirdly, NH4+ electrolytes are inexpensive and environmentally friendly. Taken together, the ammonium ion batteries (AIBs) are considered as promising candidates for practical, high-energy-density aqueous batteries. Recently, considerable efforts have been devoted to the development of cathode materials for ammonium ion storage. Ji et al. proposed a Prussian white analogue, namely, (NH4)1.47Ni[Fe(CN)6]0.88, showing a specific capacity of 60 mAh g−1 at 150 mA g−1 (0.25 ~ 1.5 V vs. standard hydrogen electrode, SHE) in (NH4)2SO4 electrolyte5. Liu et al. developed the (NH4)0.27MnO1.04(PO4)0.28 possessing a high specific capacity of 299.6 mAh g−1 at 1 A g−1 (0.2 ~ 1.2 V vs. SHE) in ammonium acetate (NH4Ac) electrolyte, with the coordination bonds between Mn atoms and acetate ions proposed to facilitate the ammonium ion storage process6. Our recent work first reported the ammonium ion storage behavior in some inorganic layered compounds including layered double hydroxides (183.7 mAh g−1 at 0.1 A g−1, 0 ~ 1.2 V vs. SHE) and layered VOPO4·2H2O (154.5 mAh g−1 at 0.1 A g−1, −0.1 ~ 1.2 V vs. SHE) with stable discharge plateau7,8.
Although remarkable progress has been made on the cathode materials for AIBs most recently, its development for practical applications is still challenged by the lack of anode materials with a low working voltage. Up to date, the development of anode materials is generally limited to transition metal oxide/sulfides (h-MoO39, h-WO310, etc.) and organic polymers (PTCDI5, PANI11, etc.). However, the low electronic conductivity of the former generally yields to unsatisfied specific capacity, and the high dissolution rate of the polymers results in poor cycling stability12. Undoubtedly, anode materials with a high capacity and excellent reversibility are in urgent need of development to construct full-cell AIBs for practical applications.
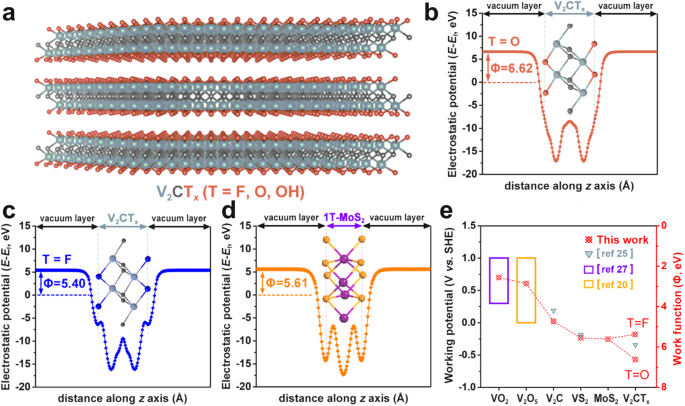
Since its first discovery and report13, MXene, a new group of two-dimensional (2D) materials with excellent conductivity and high surface activity, has been proposed to be promising electrodes for energy storage14,15,16,17. Particularly, its broad 2D channel in the layered stacking is very desirable for ion storage compared with the narrow one-dimensional (1D) channel in transition metal oxide/sulfides which is incapable of accommodating large charge carriers18,19. Considering that V-based materials have relatively low working potential in AIBs11,20,21, herein, we first carried out the theoretical simulation of V-based MXene by density functional theory (DFT) calculations using V2CTx as an example22,23. The DFT calculation result verifies that V2CTx MXene possesses the lowest working potential window compared with other V-based materials for AIBs reported, suggesting its potential as a very promising anode candidate for aqueous ammonium ion storage.
Inspired by this theoretical prediction, we tried to explore the ammonium ion storage behavior of V2CTx MXene in various aqueous electrolytes including (NH4)2SO4, NH4Cl, (NH4)2C2O4, NH4Me, NH4Ac. Surprisingly, a pseudocapacitive typed redox reaction within −1 ~ −0.01 V potential range (vs. Ag/AgCl) could be only detected in the NH4Ac electrolyte. Benefited from this pseudocapacitive behavior, our V2CTx MXene delivered a high specific capacity of 115.9 mAh g−1 (at 1 A g−1), which surpasses all of the capacitive-typed electrodes in sustainable ammonium-ion batteries up to date. The pseudocapacitive origin was investigated by an in-situ electrochemical quartz crystal microbalance (EQCM) measurement, which reveals a two-step electrochemical process. Density functional theory (DFT) simulation further verifies the formation of [NH4+(HAc)3]···O coordination bond facilitates the alternation of V2CTx terminations, thereby realizing the variation of the V valence state which significantly promotes the charge transfer in the electrochemical process. The generality of this acetate ion enhancement effect on pseudocapacitive capacity was further confirmed in the MoS2-based ammonium-ion battery system.
For more information please follow the link: https://www.nature.com/articles/s41467-024-46317-5


