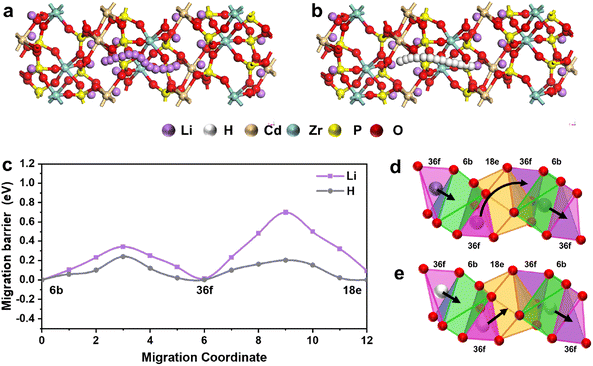
Low ionic conductivity of solid electrolytes at intermediate temperatures hinders the commercialization process of solid fuel cell technology. A sodium superionic conductor (NASICON)-structure with a rigid three-dimensional network and an interconnected interstitial space is expected to be an ideal solid electrolyte for fuel cells. Based on the H+/Li+ exchange engineering strategy, here we report a NASICON-structure proton conductor Li1+xCdx/2Zr2−x/2(PO4)3 (x = 0.5, 1, 1.5, 2) derived from CdZr4(PO4)6 to construct a fuel cell device. Among all samples, the Li3Cd1Zr1(PO4)3 cell device exhibits a high performance including peak power density 815 mW cm−2, proton conductivity 0.165 S cm−1 and activation energy 0.372 eV at 550 °C. Theoretical and experimental studies both suggest that the high proton conductivity benefits from the unique 3D interstitial space and rapid H+/Li+ exchange in the NASICON material. Under fuel cell operating conditions, the interstitial space of Li1+xCdx/2Zr2−x/2(PO4)3 (x = 2) substitutes mobile Li+ with H+ enabling fast proton transport. The new transport mechanism and excellent proton conductivity suggest that Li1+xCdx/2Zr2−x/2(PO4)3 provides new opportunities for enriching novel electrolyte materials in intermediate temperature protonic ceramic fuel cells (IT-PCFCs).Sodium superionic conductor NASICON (Na superionic conductor)-type oxide-based materials are considered to be one of the most promising solid electrolytes due to their reasonable high Li-ion conductivities at room temperature.9–11 PO4 tetrahedra and metal oxide octahedra constitute the three-dimensional connected crystal skeleton of NASICON electrolyte, and partial Li+ fills the pores of the skeleton structure to construct three-dimensional connected ion transport channels. The essential structural feature of rigid and three-dimensional NASICON materials is an interconnected interstitial space that is occupied by mobile Li+ ions.12–14 These mobile lithium ions can migrate freely and exhibit certain ion conduction properties at room temperature.
Introduction
A solid oxide fuel cell (SOFC) is an electrochemical device that can directly convert the chemical energy of hydrogen or another fuel to electrical energy through an electrochemical reaction without a conventional combustion process.1–3 The fuel cell system has been utilized in a variety of energy applications.4,5 The attractive characteristics make them quite fitting for addressing the energy crisis in the present situation of international energy development. Unfortunately, the large-scale commercialization of SOFCs is still challenging because of low ionic conductivity at high operating temperatures. Though proton conductors at lower temperatures have been proposed to promote the commercialization and future development of SOFCs,6–8 intermediate temperature protonic ceramic fuel cells (IT-PCFCs) still face the challenge of how to guarantee high performance at lower temperatures. Therefore, novel-class electrolyte materials need to be further explored and discovered to meet the more stringent requirements. Sodium superionic conductor NASICON (Na superionic conductor)-type oxide-based materials are considered to be one of the most promising solid electrolytes due to their reasonable high Li-ion conductivities at room temperature.9–11 PO4 tetrahedra and metal oxide octahedra constitute the three-dimensional connected crystal skeleton of NASICON electrolyte, and partial Li+ fills the pores of the skeleton structure to construct three-dimensional connected ion transport channels. The essential structural feature of rigid and three-dimensional NASICON materials is an interconnected interstitial space that is occupied by mobile Li+ ions.12–14 These mobile lithium ions can migrate freely and exhibit certain ion conduction properties at room temperature.
In recent years, a study on the H+/Li+ exchange engineering strategy has been proposed to turn a lithium-ion conductor into a proton conductor. Li+ ions can be substituted by H+, which implies that protons can transport through existing interstitial Li+ space to exhibit the proton conduction function.15–17 The ion-exchange strategy has been applied to fast lithium-ion conductors including Garnet18,19 and LISICON20–23 structure materials. Chen et al.20 reported that the Li13.7In0.2Sr0.1Zn(GeO4+δ)4 material was successfully converted into a proton conductor and exhibited a proton conductivity of 0.094 S cm−1 in a hydrogen atmosphere at 600 °C. Matsui et al.23 demonstrated that the Li3.13H0.37Zn0.25GeO4 conductor through the simple ion-exchange method could even obtain 5.5 mS cm−1 of electrical conductivity at 200 °C. As fast sodium-ion conductors with a similar structure, NASICON-structure materials are likely to benefit from this ion-exchange strategy. This strategy has been successfully applied to NASICON materials in reports from Stenina et al.24,25 indicating the great potential to turn NASICON materials into proton conductor electrolytes. Lu et al.26 reported that the Li vacancy generated after Li migration in NASICON materials provides the proton migration path under fuel cell conditions. However, the application of the interesting ion-exchange method on NASICON electrolytes is limited and needs further exploration.
For more information please follow the link: https://pubs.rsc.org/en/content/articlehtml/2024/ta/d3ta05182j


