Abstract
Ceramic fuel cells hold an important position for the sustainable energy future using renewable energy sources with high efficiency. The design and synthesis of active materials, interface engineering and having capability of low operating temperature is considered as an important factor to further increase the power output and stability of ceramic fuel cell devices. A novel methodology has vital importance to develop new functionalities of existing materials by introducing new different effects. The built-in electric field (BIEF) is one of the most recently used approaches to improve charge transfer and ionic conductivity of solid oxide materials. Herein, we demonstrate gradient doping strategy in CeO2–δ structure to produce BIEF effect and to modulate the proton transport effectively at the surface layer rather than bulk structure. The inclusions of La and Sr metal ions at the surface and Co-metal ions into bulk-layer of CeO2 form the gradiently doped structure. The gradient doping into CeO2 highly improves the proton transport properties through the surface layer by modifying the energy levels. Moreover, unbalanced charge distribution due to gradient doping produces built-in electric-field to provide extra driving force for protons transport through surface layer. The acquired gradiently doped fluorite structure exhibits remarkable proton conductivity of >0.2 S/cm, as a result ceramic fuel cell shows power output of >1000 mW/cm2 while operating at 500 °C. This unique work highlights the critical role of gradiently doped electrolyte in electrochemical conversion energy devices and offers new understanding and practices for sustainable energy future.
Introduction
Ceramic fuel cells (CFCs) including protonic ceramic and solid oxide fuel cells (PCFCs and SOFCs) have attracted vast attention as next-generation energy conversion and technological applications with great advantages, such as an admirable-efficiency, robust fuel flexibility and use of non-precious metal-oxide electrocatalysts. However, the high-operating temperature (typically ≥700 °C) results in high cost and low applicability of CFCs. Reducing the operating temperature of CFCs from ≥700 °C to 300–600 °C would allow the full-utilization of the existing merits of the CFCs technology. The challenge for new-generation protonic ceramic fuel cells and solid oxide fuel cells ((PCFCs and SOFCs) is to search electrolytes with decent proton and oxygen ion (H+ and O2−) conductivity. Structural doping is remained a wide-ranging methodology aimed to developing high H+ and O2−.1,2 Most often the host cations such as Zr4+/Ce4+ in fluorite structure are replaced with lower valence e.g., Y3+/Sm3+ to create deficient structure for ionic transport.3 While for developing H+ conducting electrolyte for PCFCs the vast efforts are relying on the composition of barium zirconate-based perovskite oxides, which surprisingly reveals a high area-specific ohmic-resistance (ohmic-ASR) at 300–600 °C.4 However, to achieve sufficient performance at 400–600 °C apparently looks disparate using bulk H+ and O2− conducting electrolyte in CFCs and limiting the advances of the technology.
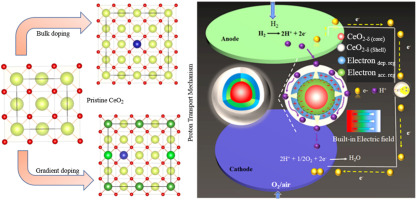
In particular, the high grain boundary resistance which is almost two orders of higher in magnitude than the grains resistance limits the ionic conductivity to reach at a sufficient level of 0.1 S/cm2 at 400–600 °C. However, this restricts the use of the existing materials with current methodologies. In this context, morphology control and surface engineering have emerged as key factors to improve the ionic conductivity of solid materials. Therefore, hybridizing the atomic orbital at the grain boundaries-region allows charge carriers delocalization and improves the charge transfer with built-in electric field (BIEF) effect.5, 6, 7, 8, 9, 10 In this way, ubiquitous crystal defects inside materials and grain boundaries (GBs) can be mobilized more efficiently.11, 12, 13, 14, 15, 16 Owning to their varied local chemical states, in this way GBs exhibit unusual mechanical, electrical, and chemical properties which may not emerge in the bulk structures. The strong interaction of H2-ceria has inspired interest to form reduced ceria (CeO2–δ) surface with defects, and H2 incorporation at the surface may start the formation of hydro-oxide species with the transfer of H2 electron to Ce 4f to form Ce3+ from Ce4+ and latterly occupy the oxygen vacancies to transport protons.17 A typical example for the deficit ceria case is illustrated by Xing et al.18 and Wang et al.,19 who found that the chemical deficits existing on CeO2 surfaces, and formed CeO2–δ surface layers, which produced a BIEF to build proton shuttles through GBs within a ceria particle pool. This phenomenon hints us that if we can take advantage of such naturally deficits and resulting surface physical properties as a rational methodology to further improve and develop the ceria surface functionalities, it would be useful for development of more advanced fuel cell applications.
In this regard, rational design of constructing heterostructure without destroying the ripple structure is an effective approach of nano-architecture to obtain the high ionic conductivity at GBs by coupling with BIEF. However, herein, we demonstrated a gradient doping methodology in CeO2 to form La0.2Sr0.05Ce0.65Co0.1O2, with a fluorite structure to exploit proton conductivity with BIEF. The structural analysis of gradient La- and Sr-doped samples in comparison to parent CeO2 and Co-doped CeO2 samples are studied. The high concentration of La and Sr cations in the shell layer could help to migrate protons/ions outward, accompanied by the formation of internal cavity (charge accumulation and depletion region), which could be explained by the “surface locking” effect. As expected, the designed gradient structure with built-in interfacial electric field could facilitate proton transfer spontaneously, exhibiting superior protonic conductivity of 0.2 S/cm and realizing a remarkable power output (>1000 mW/cm2) at low operating temperature of 500 °C with a stable operation. The results demonstrate that the employed approach could be useful in developing new set of materials with such kind of unique functionalities for advanced PCFCs/SOFCs.
For more information please follow the link: https://www.sciencedirect.com/science/article/abs/pii/S100207212300131X


